STRUCTURE AND PROPERTIES OF AMINO ACIDS
STRUCTURE AND PROPERTIES OF AMINO ACIDS:
Introduction:
Amino acids are the fundamental building blocks of proteins, which play crucial roles in various biological processes. Understanding the structure of amino acids is essential for comprehending their functions and the intricate world of proteins. In this article, we will delve into the fascinating world of amino acid structures, exploring their composition, classification, and significance.
Certainly! Amino acids can be linked together through a type of chemical bond called a peptide bond, forming chains known as polypeptides or proteins.
Amino Acid Residues:
In a polypeptide chain, each amino acid is referred to as an amino acid residue. The residues are connected by peptide bonds, which form between the carboxyl group (-COOH) of one amino acid and the amino group (-NH2) of the adjacent amino acid.
Peptide Bonds:
Peptide bonds are formed through a condensation reaction, also known as dehydration synthesis or simply peptide bond formation. During this process, a molecule of water is removed, and the carboxyl group of one amino acid combines with the amino group of the neighbouring amino acid, forming a covalent bond. This bond is called a peptide bond.
The backbone of the Chain:
The peptide bond formation creates a repeating pattern in the amino acid chain, resulting in a backbone structure. The backbone consists of the nitrogen (N) atom from the amino group, the alpha carbon (Cα) atom, and the carbonyl carbon (C=O) from the carboxyl group of each amino acid residue.
N-terminus and C-terminus:
In the polypeptide chain, the amino acid at one end is referred to as the N-terminus, while the amino acid at the opposite end is called the C-terminus. The N-terminus contains the free amino group (-NH2), while the C-terminus has the free carboxyl group (-COOH).
Side Chains (R-groups):
Each amino acid residue in the polypeptide chain has its own unique side chain, also known as the R-group. The side chain differs for each amino acid and can be nonpolar, polar, positively charged, or negatively charged, depending on the specific amino acid. The side chains extend outward from the backbone of the polypeptide chain, adding chemical diversity and functionality to the protein structure.
Primary, Secondary, Tertiary, and Quaternary Structure:
The sequence of amino acids in the polypeptide chain is referred to as the primary structure. The primary structure determines the overall shape and function of the protein. Secondary structure refers to local folding patterns within the polypeptide chain, such as alpha-helices and beta-sheets. Tertiary structure refers to the overall three-dimensional structure of a single polypeptide chain.
Section 1: The Composition of Amino Acids
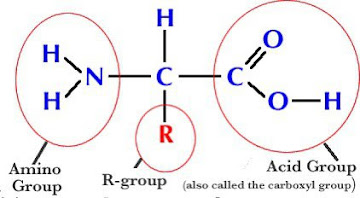
Amino acids consist of three key components: an amino group (NH2), a carboxyl group (COOH), and a side chain (also known as the R-group). These components define the chemical properties and uniqueness of each amino acid. The amino group contains a nitrogen atom bonded to two hydrogen atoms, while the carboxyl group consists of a carbon atom double-bonded to an oxygen atom and single-bonded to a hydroxyl group (OH). The side chain, varying in structure and composition, distinguishes one amino acid from another.
Section 2: Classification of Amino Acids
Amino acids can be classified into different categories based on the characteristics of their side chains. The common classifications include:
Nonpolar Amino Acids:
These amino acids have hydrophobic side chains, meaning they repel water. Examples include glycine, alanine, valine, and leucine.
Polar Amino Acids:
Amino acids with polar side chains are hydrophilic, meaning they are attracted to water. Serine, threonine, and asparagine are examples of polar amino acids.
Charged Amino Acids:
Charged amino acids can be further divided into two subcategories:
a. Positively Charged (Basic) Amino Acids:
Lysine, arginine, and histidine are examples of basic amino acids that contain positively charged side chains.
b. Negatively Charged (Acidic) Amino Acids: Aspartic acid and glutamic acid possess negatively charged side chains.
Section 3: Significance of Amino Acid Structures
The diverse structures of amino acids contribute to their varied functions within biological systems. These functions include:
Protein Synthesis: Amino acids link together through peptide bonds to form polypeptides and proteins. The specific sequence of amino acids determines the structure and function of the resulting protein.
Enzyme Catalysis: Amino acids play critical roles as catalytic residues in enzymes, facilitating chemical reactions necessary for cellular processes.
Signal Transduction: Certain amino acids act as signalling molecules, transmitting information within cells or between cells. Examples include phosphorylated serine, threonine, and tyrosine residues.
Structural Support: Amino acids like glycine and proline contribute to the structural stability of proteins, forming helices, sheets, and turns.
Section 4: General Properties and Reactions of Amino Acids
Amino acids exhibit several general properties and reactions that contribute to their biological functions:
Acid-Base Properties: Amino acids possess both acidic (carboxyl group) and basic (amino group) properties. At physiological pH, they exist in their zwitterionic form, with the carboxyl group negatively charged and the amino group positively charged.
Peptide Bond Formation: Amino acids can undergo condensation reactions to form peptide bonds. This process involves the removal of water and results in the formation of polypeptides and proteins.
Optical Isomerism: Amino acids, except glycine, exist in chiral forms as enantiomers (L- and D-forms). The L-form is predominant in proteins and exhibits specific biological activity.
Oxidation and Reduction: Amino acids can participate in oxidation and reduction reactions, which are important in cellular metabolism and energy production.
Section 5: Functional Properties of Amino Acids
Amino acids possess various functional properties that contribute to their biological significance:
Protein Structure and Function: Amino acids, when joined together, form polypeptides and proteins. The specific sequence and arrangement of amino acids determine the three-dimensional structure and function of the resulting protein.
Enzymatic Activity:
Some amino acids serve as catalytic residues in enzymes, facilitating biochemical reactions necessary for cellular processes.
Signal Transduction:
Modified amino acids, such as phosphorylated serine, threonine, and tyrosine residues, play critical roles in cell signaling pathways, transmitting information within cells or between cells.
Metabolic Intermediates:
Certain amino acids can serve as precursors for the synthesis of important biomolecules, such as neurotransmitters, nucleotides, and hormones.
Exercise:
1. Amino acids are the building blocks of proteins. Describe the general structure of an amino acid and the key functional groups present in it. 2. There are 20 standard amino acids found in proteins. Differentiate between essential and non-essential amino acids, and provide examples of each.
3. Amino acids can be classified based on their side chain properties. Name and explain the four major categories of amino acids and give an example for each category. 4. The isoelectric point (pI) is a critical parameter for amino acids. Define pI and explain how it can be calculated for a given amino acid. 5. Amino acids can exist in two isomeric forms: L-amino acids and D-amino acids. Describe the significance of L-amino acids in living organisms and why they are predominantly used in protein synthesis.
Fig.: 1- Source: (<a href="https://www.freepik.com/free-ai-image/futuristic-molecule-patterns-connect-genetic-research-discovery-generated-by-ai_41668209.htm#query=amino%20acids%20molecule%20structure&position=1&from_view=search&track=ais_ai_generated">Image By vecstock</a>)
Fig.: 2- Source: (https://www.quora.com/How-are-amino-acids-categorized-by-their-chemical-properties)
Fig.: 3- Source: (https://en.wikipedia.org/wiki/Amino_acid)


.png)
.png)
Comments
Post a Comment